- I would like to make three points regarding the article "The Exploding Gummy Bear", Chem 13 News, February 2014, pages 12 -14.)

First, you point out in the Editor's note that there are dangers involved in the "screaming gummy bear" demo. I do more or less the same demo, but using a couple of wooden splints. I tell the students that I am going to test for oxygen with a glowing splint, and then: “Oops ! It's fallen in” The result is as you see in the adjacent photo — it was taken outside on a windy day. It may not be as dramatic as the “gummy bears”, but it's still pretty dramatic for anyone who hasn't seen anything else.
Second, the authors state that
The molten potassium chlorate decomposes liberating oxygen, also an endothermic process.
2K+ClO3- + energy → 2KCl + 3O2(g)
Hard as it is to believe, this is in fact an exothermic process!
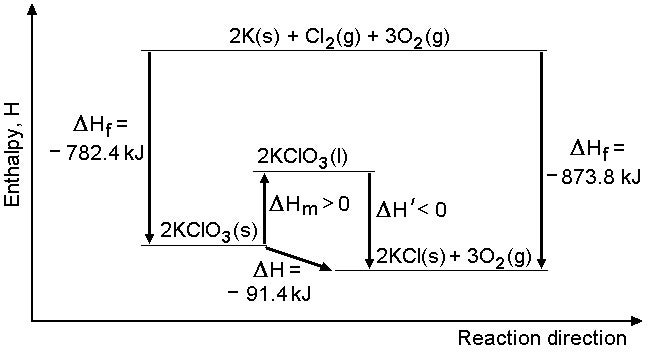
I couldn't find a figure for the heat of fusion for KClO3, but whatever it is, the heat of decomposition of the liquid is even more exothermic than that of the solid; I have assumed that the KCl remains a solid, as its melting point is much higher than that of KClO3. Presumably, the illusion of it being an endothermic process is because of the very high activation energy, necessitating continual heating, as well as the need to melt the solid in the first place.
Third, the article claims: …
each and every gummy bear is packed full of energy.
This is quite untrue! This is like saying that a liter of gasoline is packed full of energy: take it to the moon and see how much energy you'd get out of it. None! If you took to the moon a compressed or stretched spring, or a vial of nitroglycerine, then you'd have something “packed full of energy”. Perhaps what the authors could have said was something like “each and every gummy bear, on oxidation, liberates energy”.
Yehoshua Sivan
Safed, Israel
- I bet when you published the article on Gummy Bears in Chem 13 News you thought, I bet Bob responds to this.
I enclose our Supplementary Risk Assessment on this for you as well. [A link to this PDF can be found on the Chem 13 News website under “March supplemental materials”.]
That article had some items that CLEAPSS* would find rather strange.
- An Ignition tube: In the UK an ignition tube is 10 x 70 mm, the tiniest of test tubes. In our experience any borosilicate tube will do, and we used a 25 x 150 mm boiling tube. It must be borosilicate not neutral borosilicate.
- A Bunsen burner attached to natural gas is quite sufficient to melt the potassium chlorate. Unless you have no gas supply, it is not necessary to use these powerful torches.
I can see they have to be used outdoors — but it is always raining here. - I prefer potassium chlorate as it has a lower melting point.
- Because we have known the object to shoot out, the bench must be protected with heat-proof bench mats.
- We surround as much of the apparatus as possible with safety screens.
- Students should be positioned several metres away.
- We do NOT advise the use of a fume cupboard. If the fume cupboard is recirculatory, with a filter in place, sparks have been dragged on to the paper prefilter, setting the carbon filter alight.
- Both types of fume cupboards — ducted and recirculating — cannot cope with the amount of “smoke”, resulting in smoke escaping into the room.
- By the way, why are there smoke activated fire alarms in any chemistry lab? You need heat activated alarms.
- Is it smoke? I think it is condensed water vapour — which can still trigger smoke alarms — and potassium chloride.
- We use less than 15 g of potassium chlorate. The question is “Would 1 to 2 g last long enough to have the students whooping?”
- That title “Exploding?” It did not explode, it reacted vigorously. If it had exploded, glass would have scattered over everywhere.
Bob Worley
London UK
* CLEAPSS is an UK advisory service providing support in science and technology for a consortium of local authorities and their schools.







