Our Science Department enjoys planning themed activities that correspond to different holidays. Last year, we decided to make bath bombs with our classes on the last day of school before the winter holiday. This is an activity that is easy to set up, can be carried out in one class period, and has minimal clean up.
The recipe we used was adapted from The Pistachio Project website https://pistachioproject.com/2016/12/gingerbread-bath-bombs.html
Materials
(Yield: 1 dozen small bath bombs)
- ½ cup baking soda
- ¼ cup Epsom salt
- ¼ cup citric acid
- 1 tsp cinnamon
- ½ tsp ground ginger
- 2 tbsp melted coconut oil (extra if needed)
- ½ tsp molasses (1 tsp if you want a darker colour)
- silicone baking molds
- large bowl
- measuring cups/spoons
Procedure
- Prepare the silicone molds by rubbing melted coconut oil into them or spraying with a nonstick cooking spray.
- Mix all ingredients into a large, dry bowl, using a spoon at first and moving to hands to make sure that the ingredients are holding together. If they are not, then you will need to add more melted coconut oil, ½ tsp at a time, until they do.
- Press the mixture into the prepared silicone molds, making sure to firmly pack the mixture in.
- Leave the molds overnight to dry, or alternatively, place the silicone molds into a freezer to allow them to set quickly within 20 to 30 minutes.
- Carefully loosen the bath bombs from the molds by stretching the silicone before popping them out. The bath bombs could be placed in a variety of containers or wrapped and decorated as a gift.
Safety concerns
As this is a “food” lab, there are some safety issues that should be considered if carrying out this activity in a Chemistry Lab. Students need to wash their hands before starting and we cover all lab bench surfaces with dollar store tablecloths. The ingredients, measuring cups, bowls and spoons are always stored outside of the lab, as they are for food use only.
Where’s the chemistry?
In bath bombs, once water is added, the citric acid reacts with the baking soda to produce sodium citrate and carbonic acid, which further decomposes into water and carbon dioxide.
3NaHCO3(aq) + C6H8O7(aq) → Na3C6H5O7(aq) + 3H2O(l) + 3CO2(g)
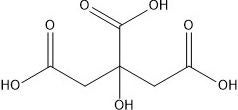
citric acid
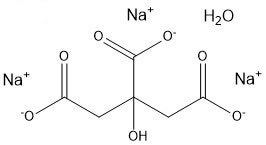
sodium citrate
During cleanup I like to have the students watch as they rinse out the bowls. This gives students an opportunity to discuss what ingredients are involved in making the bath bombs fizz, what gas is produced, and the type of chemical reaction. There is a very detailed description of the two chemical reactions involved, saponification and the neutralization reaction on this website — https://kashifkhansbathbomb.weebly.com/chemical-reactions.html.
Variations
With so many bath bomb recipes available, this lab could be easily adapted for a Valentine’s Day activity or other seasonal events by changing the colour and scent of the bath bombs.







