(This is a reprint from the September 2007 issue of Chem 13 News, pages 4-5.)
When a 10.0-g sample of a mixture of CH4 and C2H6 is burned in excess oxygen, exactly 525 kJ of heat is produced. What is the percentage by mass of CH4 in the original mixture?
The molar mass for CH4 is 16.042 g mol-1; and the molar mass for C2H6 is 30.068 g mol-1.
![]()
∆H = −890.4 kJ (per mol CH4)
![]()
∆H = −1560.0 kJ (per mol C2H6)
Only 17% of students answered this question correctly on the 2007 CHEM 13 NEWS Exam. (Interestingly, 17% is also the answer to the question!!)
It has been our experience that many students struggle with stoichiometry problems involving mixtures, particularly when two components in the mixture contribute to the amount of product (or heat) obtained. A common error made by many students when solving a problem of this type is that they add the two chemical equations together to obtain a single chemical equation involving both components of the mixture. If the two chemical equations given above are added together, we obtain the equation below.
![]()
∆H = −2450.4
While the chemical equation above is balanced, it is wrong! It implies that both CH4 and C2H6 must be present, and furthermore that they must be present in a 1:1 ratio in order to produce CO2 and H2O. It should be perfectly clear from the chemical equations that were given that CH4 will react with O2 to give CO2 and H2O, whether or not any C2H6 is present. (Similarly, C2H6 will react with O2 whether or not any CH4 is present.) The two combustion reactions are independent reactions and must not be combined, no matter how tempting it might be to add them.
Perhaps many students do not fully appreciate the difference between simultaneous reactions and consecutive reactions. Consecutive reactions are reactions that occur sequentially to yield products, whereas simultaneous reactions are a set of reactions that occur simultaneously and independently of each other. An example of a set of consecutive reactions is given below for the step-wise oxidation of HBr:
HBr + O2 → HOOBr (1)
HOOBr + HBr → 2 HOBr (2)
HOBr + HBr → H2O + Br2 (3)
Evidence that the three reactions (1)-(3) form a sequence is the fact that a product from an earlier reaction is a reactant in a subsequent reaction. For example, reaction (2) cannot occur until HOOBr is produced by reaction (1), and similarly, reaction (3) cannot occur until some HOBr is produced by reaction (2). The chemical equations for a set of consecutive reactions can be added algebraically to obtain a chemical equation for the “net” or “overall” process. For the set of reactions given above, the correct combination is (1) + (2) + 2×(3), which gives the result below.
4 HBr + O2 → 2 H2O + 2 Br2 (4)
Note that equation (3) must be doubled before the equations are added together. Although the overall chemical equation (4) “hides” some of the chemistry involved, it correctly shows that HBr and O2 are consumed in the ratio 4:1 and that H2O and Br2 are produced in a 2:2, or 1:1, ratio.
The combustion reactions for CH4 and C2H6 are not consecutive reactions; they are simultaneous reactions. Simultaneous reactions must never be added together. The amount of product (or heat) obtained from each reaction is calculated independently, and the total amount of product (or heat) is calculated by adding the two amounts together at the end, as shown below.
Approach #1:
We are asked to determine the amount of CH4 in the sample, so let “x” be the mass of CH4. The mass of C2H6 must be 10.00 − x. Therefore, the number of moles of CH4 is (x/16.042) and the number of moles of C2H6 is (10.00 − x)/30.068.
The combustion reactions tell us that we get 890.4 kJ of heat for every mole of CH4 and 1560.0 kJ of heat for every mole of C2H6. If q1 and q2 represent the amounts of heat we get from (x/16.042) moles of CH4 and (10.00 − x)/30.068 moles of C2H6, respectively, then we have:
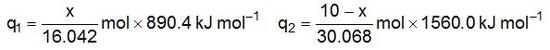
The total amount of heat is q1 + q2 = 525 kJ, and so:
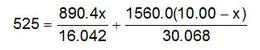
Solving for x, we obtain x = 1.72 and mass percentage of CH4 in the original mixture is approximately 17%.
Approach #2:
We first calculate the amounts of heat that would be obtained if the sample were pure CH4 or pure C2H6:
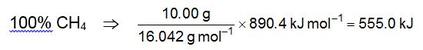
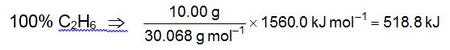
If “x” represents the fraction of the total mass that is CH4, then q1 = 555.0x and q2 = 518.8(1−x). Therefore:
525 = 555.0 x + 518.8 (1 − x)
x = 0.17
Therefore, the sample is 17% CH4 by mass.
Actually, these two approaches are only superficially different. Both approaches consider how much heat is obtained from each reaction and those two quantities are then added together and set equal to the total amount of heat. The difference between the two approaches is only in what “x” represents. Another approach is to let “x” and “y” be the number of moles of CH4 and C2H6, respectively. The mass of the sample is 16.042x + 30.068y = 10.00 and the quantity of heat released is 890.4x + 1560.0y = 525. In this approach, one must solve two equations in two unknowns.
Consider applying one of these approaches to the following problem, which is slightly more complicated than problem #35 on the 2007 CHEM 13 NEWS Exam.
A 2.00-g sample of a mixture of CaCl2 and RbCl is treated with excess AgNO3(aq), causing AgCl(s) to precipitate from the solution. If the mass of AgCl obtained is 3.45 g, then what is the percentage by mass of RbCl in the original mixture? (Answer: 61.4% RbCl)






