“Plop, plop, fizz, fizz”, sings Speedy about the wonders of the antacid Alka-SeltzerⓇ in a 1950s television advertisement.1 The “fizz” is carbon dioxide gas, which is mainly produced by the reaction of sodium bicarbonate with citric acid. A trip to a store will show that this reaction can be found in several consumer products, including other antacids and denture cleansers. In addition, many chemistry teachers have designed activities around the reaction of sodium bicarbonate with citric acid.2-4 Since the antacid labels include the masses of the reactants, I have used the reaction as a mini-lab for students to practice their stoichiometry calculations on a consumer product. Students measure the mass of a container of water and the antacid before and after the reaction. The actual yield of carbon dioxide is calculated as a difference between the two masses. Students use information on the label to determine the theoretical yield of carbon dioxide.
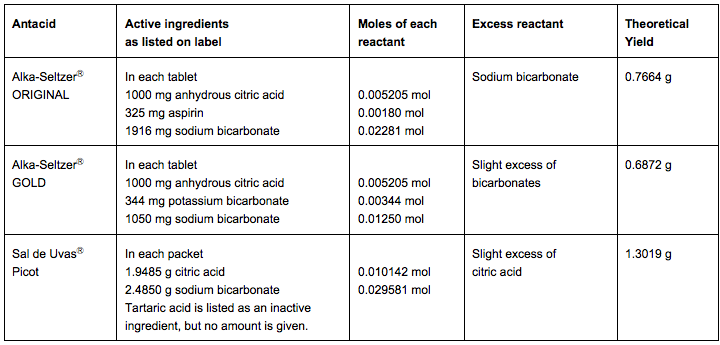
In addition to the original Alka-SeltzerⓇ, this activity can be done with other antacids. The active ingredients listed on the labels are shown in the following table. Only Alka-SeltzerⓇ ORIGINAL has sodium bicarbonate in excess. The active ingredient in all three formulations is sodium citrate, along with potassium citrate for Alka-SeltzerⓇ GOLD.
Chemical reactions in antacids
Since a molecule of citric acid contains three carboxylic acid groups, 3 moles of sodium bicarbonate react with each mole of citric acid.
3NaHCO3 + C6H8O7 → Na3C6H5O7 + 3H2O + 3CO2
A molecule of aspirin contains only one carboxylic acid group giving a 1:1 mole ratio of sodium bicarbonate to aspirin.
NaHCO3 + C9H8O4 → NaC9H7O4 + H2O + CO2
References (websites accessed December 2016)
- Alka-seltzer Vintage Television Commercial, YouTube, https://www.youtube.com/watch?v=J5G4USWB79c
- M.E. Harris, B. Walker, JCE Classroom Activity: #58, Bath Bubblers, Journal of Chemical Education, 80, 2003, page 1416B.
- B. Rohrig, JCE Classroom Activity: #31, Fizzy Drinks: Stoichiometry You Can Taste, Journal of Chemical Education, 77, 2000, page 1608B.
- Alka-Seltzer Science Experiments, Retrieved from https://www.alkaseltzer.com, June 2016.






