When discussing ionic solutions, it is common for a student to ask:
“How do you know how many particles an ionic compound breaks into when dissolved in water?”
Students find the concept of having 1 mole of NaCl in solution, resulting in 2 moles of ions — Na+ and Cl- — difficult.
I came up with the plastic Easter egg idea to illustrate this concept. We started with a dozen plastic eggs in the basket. When we put these eggs into “solution” or beaker, the eggs were split into two halves. This illustrates NaCl breaking into twice as many particles. There are now 24 halves or particles.
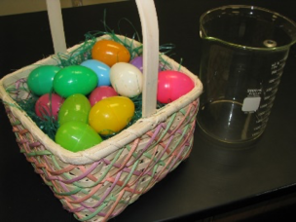

As you put the plastic eggs into the beaker they are taken apart.


All the units are made of three (two yellow and one blue) Lego bricks. As units are placed in the beaker they are taken apart.
I asked students “what could be used to illustrate this relationship for an ionic compound like MgCl2?” They suggested LEGO® building bricks. We built one dozen MgCl2 formula units composed of one 2x1 brick and two 1x1 bricks. As the units are added to the beaker — in solution — these units are broken into the three pieces — 3 dozen ions.
The Periodic Table of Videos people from the School of Chemistry at The University of Nottingham, United Kingdom have a fun chemistry video putting Easter Cream Eggs to the test. The video is around seven minutes long with several possible tie-ins to the high school curriculum. One great thing about this video is the giddy enthusiasm of the demonstrators.There are three experiments with the Easter Cream Eggs.
- Easter Cream Egg in liquid nitrogen
- Easter Cream EggPlaced in molten potassium chlorate — imagine the flaming gummy bear on a much larger scale. In the video the evaporating dish cracks during this reaction. This demo is not recommended for a classroom.
- Easter Cream egg placed under vacuumThis demonstration can be done in the classroom for those who have a vacuum desiccator. Students will enjoy watching the expanding, oozing cream coming out of the chocolate egg.



Google “Periodic Table of Video” and “Easter Cream Eggs”.
Student questions after the video
Explain why the creamy filling inside the Easter Cream Egg expands when it is under vacuum.
Have students balance the chemical equation of the Easter Cream Egg reacting with the molten potassium chlorate.
Unbalanced equation:
C12H22O11(s) + KClO3(s) → CO2 (g) + H2O (g) + KCl(s)
Why does the KClO3 need to be heated before the Easter Cream Egg is added to the evaporating dish? What gas is liberated?
What is the temperature of the liquid nitrogen?
Why does the “frozen” Easter Cream Egg start to appear white soon after it is removed from the liquid nitrogen?






