This is the 10th article in the series, which considers some common organic molecules encountered in our everyday lives. Described will be some general chemical information about the organic molecule, how it is useful to us, and other interesting facts.
Hexenal
Chemical name: cis-3-hexenal or (Z)-hex-3-enal
CAS registry number: 6789-80-6
Formula: C6H10O
Structure: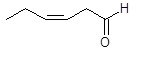
This colourless liquid is an aldehyde and is only slightly soluble in water, but is miscible with ether and ethanol. It is believed to be a component of the odour of freshly mowed grass. The smell does not last long because cis-3-hexenal is unstable and rearranges to form trans-2-hexenal. This molecule is an isomer of cis-3-hexenal but is more stable and differs in the stereochemistry and position of the double bond. Trans-3-hexenal, another isomer, can also form. These naturally occurring aldehydes are believed to be produced in plants, and grass in particular, as protection from bacteria when cells are injured to give time for the cut ends to heal. The compounds, trans-2-hexenal and trans-3-hexenal have been found to be active against a range of bacteria, including Salmonella choleraesuis which is the most common cause of sepsis.1
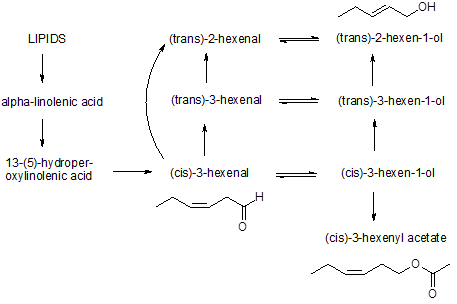
So how does the “freshly cut grass” end up producing (cis)-3-hexenal? It arises from rapid enzymatic oxidation/breakdown of linolenic acid present in the cells of grass leaves, resulting in highly odoriferous C6 compounds, such as (cis)-3-hexenal, (cis)-3-hexen-1-ol and (cis)-3-hexenyl acetate. These chemicals aren't present in the intact leaves but are formed in very small amounts when the grass leaves (and leaves of many other plants) are cut.2 These compounds are volatile and extremely odour intensive.
For a chemical to be perceived by our nose it must be small in size, lipophilic and volatile. An odour threshold is defined as the lowest concentration of a vapour in air that can be detected by smell and cis-3-hexenal has a very low odour threshold of 0.25 parts per billion (ppb). It readily rearranges to the more stable trans-2-hexenal, which has a much higher odour threshold (17 ppb). An even higher odour threshold (70 ppb) is found for the related alcohol cis-3-hexen-1-ol.2 Even so, this alcohol — sometimes called the leaf alcohol — is used quite extensively in the perfume industry to give fragrances a natural, fresh, clean scent or what perfumers call “green odours”.
 Researchers have actually discovered that the compounds released when mowing your lawn make people feel happy, relaxed , and could prevent mental decline in old age. They claim it works directly on the brain, particularly the emotional and memory parts.3,4 People have a very strong association between memory and the sense of smell, much more so than the other senses. Olfaction is closely linked with the limbic system, which is responsible for emotions and memory. The sense of smell has a direct link to the cerebral cortex in the brain; messages involving other senses such as touch or taste have a more circuitous route. From his research, Dr. Lavidis and his team have developed a perfume that “smells like a freshly-cut lawn” and reportedly relieves stress and helps boost memory.5
Researchers have actually discovered that the compounds released when mowing your lawn make people feel happy, relaxed , and could prevent mental decline in old age. They claim it works directly on the brain, particularly the emotional and memory parts.3,4 People have a very strong association between memory and the sense of smell, much more so than the other senses. Olfaction is closely linked with the limbic system, which is responsible for emotions and memory. The sense of smell has a direct link to the cerebral cortex in the brain; messages involving other senses such as touch or taste have a more circuitous route. From his research, Dr. Lavidis and his team have developed a perfume that “smells like a freshly-cut lawn” and reportedly relieves stress and helps boost memory.5
Humans can distinguish between over 10,000 different smells. Molecules from objects around us evaporate and travel to our noses, where they activate cells in the olfactory epithelium (a patch of tissue about the size of a postage stamp located high in the nasal cavity). Each cell has receptors that have a certain shape to fit a certain molecule, like a lock and key. When you smell something, the receptor gets activated and sends a message to your brain. It is interesting to note that small changes in the chemical structure of many organic compounds can produce distinct smells.5 The fine-tuning of our receptor machinery allows us to distinguish between different molecules. We can distinguish between different functional groups, the exchange of aliphatic and aromatic rings, and even elongation of a carbon chain.6 This is evident with cis-3-hexenal and trans-2-hexenal — isomers having noticeably different odour thresholds. Many fragrances are derived from plants, and plant products can be used to demonstrate how small changes in the chemical structure of an odorant can give rise to completely different smells or at least distinguishable flavours.
The science of scent would be a great way to introduce isomers to your organic class. The leaf aldehyde also helps debunk the popular myth that a “chemical smell” is bad. One exercise you can do with your students would be to have them match a chemical to its smell as a way to remember their functional groups. You might also want to have them go smell a freshly mowed lawn before writing a test as this should help them relax and boost their memory.
References (Websites accessed April 2013.)
- I. Kubo, K. Fujita, A. Kubo, K. Nihei, and T. Ogura, Journal of Agricultural and Food Chemistry, 52, 3329, 2004.
- A. Hatanaka, “The Biogeneration of Green Odour by Green Leaves”, Phytochemistry. Vol 34, No 5, pages 1201-1218, 1993.
- http://www.serenascent.com.au/index.php
- B. Malnic, J. Hirono, T. Sato, L. Buck, Cell, 96, pages 713-723, 1999.
- P.T. Choy and N.A. Lavidis, “Praescent attenuates the up-regulation of sympathetic neurotransmission induced by chronic stress,” in Proceedings of International Brain Research Organization. July, 2007.
- http://www.scienceinschool.org/print/378.






