Introduction
Your students may find it interesting to compare the acid content of a low-acid apple juice or a low-acid orange juice to that in a regular juice of the same brand. These sample types could be added to an existing acid-base titration experiment, form the basis of a new experiment, or be used as project topics.
Low-acid fruit juices?
A regular apple juice and a low-acid apple juice of the same brand — Rougemont™ McIntosh and Mellow/Low-Acid apple juices1 — were amongst the samples purchased at a Metro grocery store in Toronto for student trials of a grade 7 drop-count acid-base titration experiment.2a It seemed reasonable to expect that these two juices would differ measurably in acid content. Surprise! The student drop-count trials found little or no difference in the acid content of these two apple juices.
To confirm the student results, the experiment was repeated as a precise gravimetric titration2b with new samples of the two apple juices. For comparison, Minute Maid™ brand Original and Low-Acid frozen concentrated orange juices3, made up according to the instructions, were also titrated. The pH of each juice type was also measured. The results are in Table 1. The gravimetric titrations with 0.1 M NaOH solution were performed using phenolphthalein indicator in 125-mL Erlenmeyer flasks. All of the titrations were performed in the same session with the same NaOH solution.
Table 1: Data for gravimetric titration of 5-mL samples of apple and orange juices with 0.1 M NaOH
| Trial | Rougemont McIntosh | Roguemont Mellow | Minute Maid Original | Minute Maid Low-Acid |
| 1 | 2.92 g | 2.82g | 5.43 g | 4.28 g |
| 2 | 2.97 g | 2.84 g | 5.45 g | 4.38 g |
| 3 | 2.95 g | 2.93 g | 5.50 g | 4.17 g |
| Mean | 2.95 g | 2.86 g | 5.46 g | 4.25 g |
| Acid content | 3.96 g/L Malic Acid | 3.84 g/L Malic Acid | 6.99 g/L Citric Acid | 5.48 g/L Citric Acid |
| pH | 3.43 | 3.46 | 3.63 | 3.93 |
Each 5-mL sample of apple juice or orange juice was measured out using a relatively small-bore 10-mL graduated cylinder to enhance precision. The indicator end-point was slightly less distinct for the orange juices than for the apple juices, due to the presence of solid pulp.
The data required for calculating the acid content of each juice from its titration result are found in Table 2. By convention, the acid content of an apple juice is reported as malic acid, which is the major acid present. Similarly, the acid content of an orange juice is reported as citric acid. A sample calculation of the g/L concentration of acid in a juice is found in reference 2b. A 5-mL sample of juice having a titration volume of 2.5 g to 6 g of 0.1 M NaOH solution is suitable for a gravimetric titration.4 If you are using buret technology, a larger titration volume may be preferred. This can be achieved by using a larger sample size or a more dilute NaOH solution.
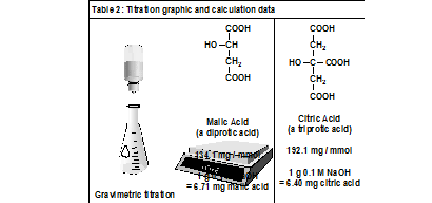
Explanation of the analysis results
The two orange juices differed in acid content by 24%, which seemed reasonable. The two apple juices differed by only 3%, which seemed strange. My query to A. Lassonde Inc. produced this response from Maxime Salvail, Chemiste, which I quote:
“..I am a chemist working for Lassonde and I was forwarded your question about mellow apple juice acidity. Your observations are true and are considered as normal, here is the explanation:
The “mellow” claim means this juice has less acidity when compared to a NFC (not from concentrate) juice. NFC usually have high acidity and depends only on the apples, if they were picked early or not in the season, etc.
On the other side, McIntosh juice can be made from concentrate or NFC or even in combination, and therefore can have a broad range of acidity from lot to lot.
Here are some specifications: Mellow: 0.35% to 0.45%;
NFC: up to 0.65%; McIntosh: 0.35% to 0.65%
(all expressed as malic acid)..”
The specification % values are in g/100 mL units; multiply by 10 to get g/L values. According to the manufacturer’s specifications, the “Low-Acid” juice can be higher in acid content than the regular juice. My conclusion is that their “Low-Acid” juice would be more truthfully labeled “Not-High-Acid” juice.
As well as producing the Rougemont juices, A. Lassonde Inc. also markets juices under the Allen’s™ brand, and probably produces the Selection™ house-brand apple juices for the Metro grocery chain. Both of these latter brands also have a low-acid version. I have no data for the latter brands, but expect them to be similar to the Rougemont. Look for similar products in your local grocery stores.
In case you are thinking of taste testing different apple and orange juices, it takes a highly trained taster to distinguish the acidity level in the presence of the high levels of sweetness in most juices.5
Questions for students
- For what reason(s) would a consumer want a low-acid juice?
- Are there any regulations or laws where you live that apply to label claims of being a “low-acid” juice?
- How can a regular apple juice be chemically converted to a low-acid juice in a manner that is safe for consumption?
- Why is the pH of the orange juices higher than that of the apple juices, even though the amount of base required to neutralize the acid contents is greater?
References
- Rougemont: Click on: a. McIntosh apple; b. Mellow apple.
- Articles available from the web page: http://www.uclmail.net/users/dn.cash/articles.html
- Drop Count Titration (16.3 MB):
http://uclmail.net/users/dn.cash/DropCountTitr.pdf - Gravimetric Titration 3 (2.1 MB):
http://www.uclmail.net/users/dn.cash/GravTitr3.pdf
- Drop Count Titration (16.3 MB):
- Minute Maid: http://www.minutemaid.com/products/oj/default.html
Click on: a. Original Orange Juice; b. Low Acid Orange Juice. - David Cash, “Gravimetric titration: simple, buret-free, and high precision”, Chem 13 News, December 2012/January 2013, pages 12-14.
- Peynaud, Émile, The Taste of Wine: The Art and Science of Wine Appreciation, trans. Michael Schuster, London, Macdonald Orbis, 1996, ISBN 0-471-11376-X, pages 149-152. Also: http://en.wikipedia.org/wiki/Émile_Peynaud.






