We snapped a quick photo of Sue Stathopulos unpacking her new thermometers.
A few blocks from my home a parcel of land is for sale with a large sign advertising its size as “+/- 5.23 acres”. Is it possible that I could purchase -5.23 acres of land? Would the seller then pay me for this purchase? Of course this is ridiculous, and we could logically assume the vendor isn’t selling negative acreage, and so interpret the sign to mean approximately 5.23 acres, but this lack of clarity is not acceptable in science.
The numbers and values we use have distinct meaning, and thus must be presented in a way that is clear to others. The use of significant figures in our data is a simple way to do this in undergraduate chemistry labs, so we work hard to ensure students understand how to use significant figures properly. The previous sentence should be interpreted as “we deduct marks for incorrect significant figures, so that there is an incentive for students to learn how to present data properly.”
It is often surprising to first year students to have marks deducted for incorrect significant figures in their lab reports. Students hear from friends that significant figures are important, and I often field emails asking “how many significant figures should my answer have?”
I typically respond vaguely with something like “well, that depends; how many significant figures did you record in your data?” Because that truly is the heart of the matter: the measurements made, and the measuring tools used, limit the accuracy that can be reported in experimental results.
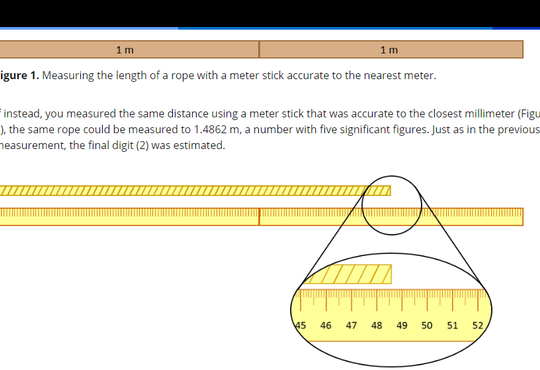
But how do we get students to see, and more importantly, understand this notion? Oh, the times I have observed students struggling with volume measurements: adding a few extra drops with a dropper, then pouring a little solution out, getting their eyes level with the meniscus to check, adding a few more drops, etc… all while using a beaker as the measuring tool! Yes, a beaker! With an accuracy of +/- 5%!
We indicate that “every value in your lab manual is written so that all of the digits are significant” hoping that students will see a volume like 10.00 mL and recognize that it will need to be measured with something like a volumetric pipette, not a beaker or graduated cylinder. But for many students this is not intuitive, not yet… but perhaps with enough practice…
With great optimism and some “flipped lab” ideas for the upcoming year I plan to target two simple tasks, getting students to:
- use the correct measuring tool for the task
- record the measured value with the appropriate significant figures.
The aim is to make recording and reporting significant figures correctly second nature to our students, hopefully setting them up for success in whatever field they pursue. Except maybe real estate…
Sue Stathopulos is the instructor of First Year Chemistry Labs at the University of Waterloo, who is forever an optimist and in general awe of the brilliant students she encounters. This year over 1,600 students will be experimenting in Sue’s undergraduate chemistry lab. In Part 2 Sue will share some of her flipped initiatives.)