The cost of everything keeps going up! What’s a laboratory science instructor to do when it comes time to place an order for chemicals and equipment for the next year? One solution is to find cheaper substitutes that can still achieve the desired student outcomes.
A recent paper1 described a lab activity involving the stoichiometry of antacids, one of which was Alka-Seltzer. I would like to describe some of the chemical principles that can be illustrated with the use of Alka-Seltzer.
I hope that teachers will find the following classroom and laboratory exercises interesting enough to try some with their own students. The exercises can be used from elementary school right up through first-year university chemistry courses.
Part 1. Plop, plop, fizz, fizz
Kids of all ages are fascinated by the evolution of a gas. John Fortman has described several uses of carbonated soft drinks and the use of dry yeast powder with hydrogen peroxide.2-4 Dropping an Alka-Seltzer tablet into water sets in motion the wonderful display of carbon dioxide bubbles. One can use a small test tube with a balloon attached to the top and rapidly collect a sample of carbon dioxide gas. You will now have a sample that can be used to extinguish a candle or a burning splint to illustrate that the gas will not support combustion. This is a much safer method for younger students than the action of hydrochloric acid on marble chips. By using several tablets in a tall graduated cylinder containing several millilitres of soap solution one has a safer alternative to the “elephant’s toothpaste” demo,5 which uses concentrated hydrogen peroxide solution. The evolution of the carbon dioxide gas will propel the soap suds up the graduated cylinder. You can also place the tablet into a beaker of water containing pieces of rice and a raisin or two and watch them be lifted to the surface of the water and then fall back down once the adhering bubbles of carbon dioxide are dislodged.
Part 2. Thermochemistry
I have written earlier regarding the confusion experienced by some students with the difference between kinetic and thermodynamic stability.6 If you have observant students, some of them may notice that the temperature of the water drops as the Alka-Seltzer tablet dissolves; i.e., an endothermic process. Heat was absorbed from the solution at the same time as the evolution of the gas was taking place. Now show students, the decomposition of hydrogen peroxide with and without a catalyst. The evolution of oxygen gas from hydrogen peroxide solution is an example of an exothermic process. It is useful to point out that there is no direct connection between the sign of ∆H and the evolution of a gas. This provides an excellent way of easing the confusion between kinetic and thermodynamic stability. A related demonstration involves the use of boiling water and a balloon to illustrate the differences between the terms “heat” and “work”.7 Reactions can be classified as either endothermic or exothermic (q term), as well as either endergonic or exergonic (w term); the combination of the two terms is a mathematical expression known as the 1st Law of Thermodynamics (∆E = q + w).
Part 3. Kinetics
Most reactions proceed faster as the temperature increases. Is the same true for dropping an Alka-Seltzer tablet into water? Indeed it is!! Depending on the temperature of the water, the tablet will stay on the bottom of the container until the reaction is finished (very cold water), or float on top of the water through-out the entire reaction (warm water). At a temperature in between the two extremes, the tablet will start off on the bottom of the container, then as its mass decreases, gradually move to the surface of the water. Crushing the tablet into a fine powder can also be used to illustrate the effect of surface area on the rate of a reaction. A much safer (although not as spectacular) demonstration than blowing flour into a Bunsen Burner flame!!
Part 4. Determination of Ea
Some students may initially expect that exothermic reactions should proceed much faster than endothermic reactions, but this is clearly not the case. The exothermic conversion of gasoline to the combustion products CO2 and H2O is extremely rapid, but the reaction takes place only after the gasoline has been ignited. We can classify gasoline as being thermodynamically unstable relative to the products of combustion (exothermic process), but kinetically stable (reaction is very slow in the absence of a spark). The rate at which a reaction takes place is a function of the height of the energy barrier between reactant and product. This barrier is known as the activation energy (Ea) and the reactant molecules must collide with sufficient energy (and the correct orientation with respect to each other) to surpass the barrier. Increasing the concentration of the reactants will result in a greater number of collisions and therefore a faster rate of reaction. Raising the temperature will also lead to a greater number of collisions and therefore a faster rate of reaction. The rate of any reaction involving a substance X can be written as:
![]()
where k is the rate constant for the process, and y is the order of the reaction. In the reaction of the Alka-Seltzer tablet with water, one can make the assumption that the rate of the reaction is first-order. The rate of the reaction can be measured by observing the time (tx) taken for the Alka-Seltzer tablet to disappear. We can also assume that the rate constant for the process is proportional to 1/tx. The relationship between the rate constant (k) and the activation energy (Ea) is as follows:
![]()
where A is a constant (the frequency factor), T is the absolute temperature, and R is the gas constant (8.314 J K-1 mol-1).
The students should realize that this equation is in the form of the general expression for a straight line, i.e., y = mx + b where y = ln k and x = 1/T. Since 1/tx is proportional to the rate constant k, a plot of ln (1/tx) versus 1/T should produce a straight line which will have a slope equal to -Ea/R.
Determination of Ea for the process
Place 500 mL of distilled water in a 1000 mL Erlenmeyer flask and determine the temperature of the water. The volume of water and size of the container are not critical. Drop an Alka-Seltzer tablet into the water and determine the number of seconds it takes (tx) until the tablet has completely disappeared.
Repeat the process for a total of five different water temperatures. You should arrange to have readings for water temperatures between 1° and 30°C. The tablet will merely bubble on the surface of the water if its temperature is above 40°C.
|
Sample student measurements |
|
|
Temp °C |
Time (s) |
|
2.8 |
130 |
|
13.2 |
90 |
|
19.3 |
44 |
|
33.4 |
31 |
|
42.2 |
21 |
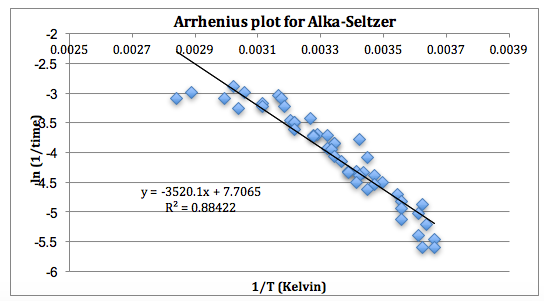
Using the data, prepare an Arrhenius plot with ln 1/tx on the y-axis and 1/T (Kelvin) on the x-axis and then use the slope of the line to determine the activation energy (Ea) for the process. The results from one class are shown on the following Arrhenius plot.
Using the slope from the graph, a value of 29 kJ is obtained for the activation energy (Ea). The line of best fit shows systematic errors in the lab and can lead to a great discussion. You will notice that the temperature range from 100°C to 40°C (1/T = 0.0027 to 0.0032 1/K) has more variance, likely due to the tablet rising to the surface of the water as noted above.
I hope that I have provided a brief glimpse of the many possible ways in which Alka-Seltzer tablets may be used in the classroom/laboratory. Please try one or more of them with your students, and I would love to hear how they respond.
References
- P. Dwyer-Hallquist, Stoichiometry of antacids Chem 13 News, February 2017, (issue 428), page 9.
- J. Fortman, Chem 13 News, February 1995, 237, page 10.
- J. Fortman, Chem 13 News, January 1995, 236, page 13.
- J. Fortman, Chem 13 News, November 1994, 234, page 7.
- R. Perkins, Chem 13 News, April 2011, 382, page 7.
- R. Perkins, Chem 13 News, December 1991, 208, page 1.
- R. Perkins, Chem 13 News, September 1993, 223, page 26.
[If you want an article from a past issue of Chem 13 News, email us for an electronic copy — Chem13News@uwaterloo.ca. When you contact us for articles, it helps us know what teachers are interested in reading about -- please feel free to ask!]






