The invention of the spectroscope by Joseph Fraunhofer (1787-1826) in 1818 was nothing short of a game changer. Since the 1500’s, alchemists knew that salts could impart various colours to a flame; however Fraunhofer showed that by measuring the exact wavelengths of these colours, each element emitted its own spectral pattern, much like a fingerprint.
In 1859, Robert Wilhelm Bunsen (1811-1899) and Gustav Robert Kirchhoff (1824-1887) developed the modern version of this instrument called a flame spectroscope, which allowed them to precisely identify elements by their emission spectra - even new elements within mixtures and compounds. Four elements were discovered using this instrument and they were all named for their flame test colors: red (rubidium), sky blue (cesium), green (thallium), and indigo (indium). Chemists could now detect small quantities before weighable quantities of these elements were actually prepared, heralding a new age in elemental analysis on Earth and in distant stars.
Read more: "Spectroscopy," by James Marshall, a Chem 13 News article (October 2019).
1855-1864: cesium, rubidium, thallium, indium
Cesium, 55

Toronto, Ontario, Canada
Teacher: Salini Kanaesu
Artist: Sola Needham
I focused on the discovery and characteristics of cesium. Pencil crayon was used to draw Robert Bunsen and Gustav Kirchhoff, the chemists who discovered it spectroscopically. While analyzing mineral water they found cesium, displayed by two sky-blue lines in its spectrum. This is symbolized on the right side of the artwork. I also drew cesium in its semi-solid crystal form with a thermometer displaying a low temperature, as cesium has a low melting point. I also drew cesium reacting with water vigorously. The resulting smoke is in the shape of Germany with a mark for Heidelberg where cesium was discovered.
Rubidium, 37

Naples, Florida, U.S.A.
Teacher: Michelle Joyce
Artists: Logan G., Lindsay L., Madison P.
The ruby red background was used to reflect the name rubidium. The element was discovered in 1861 by Robert Bunsen (as represented by the Bunsen burner) and Gustav Kirchhoff. The two deep red lines in the atomic spectra were a new discovery and helped in the identification of this element for the first time.
Mixed media including paint, pencil, and ink were used to create the artwork for rubidium. There were multiple artists contributing different parts of the element representation.
Thallium, 81

Winnipeg, Manitoba, Canada
Teachers: Angela Russenholt, Barb Bell
Artists: Payton Chan, Angelina Clemente, Sage Cournoyer, Kaytlin Nichols, Kyla Soriano, Alexus Wong
Our tile represents thallium’s chemical and physical properties using colours and symbols. The coloured stripes on the black background represent its spectral emission lines. British chemist William Crookes discovered thallium spectroscopically in 1861. The branch and leaves represent the origin of the name thallium, which derives from “thallos” (Greek for a green shoot or twig). The leaf with a skull represents the fact that this element is extremely toxic and should be handled with care. The other leaves show thallium’s atomic number and green flame test colour. We chose to represent thallium’s symbol with a silver foil-like material because it resembles its actual appearance.
Indium, 49
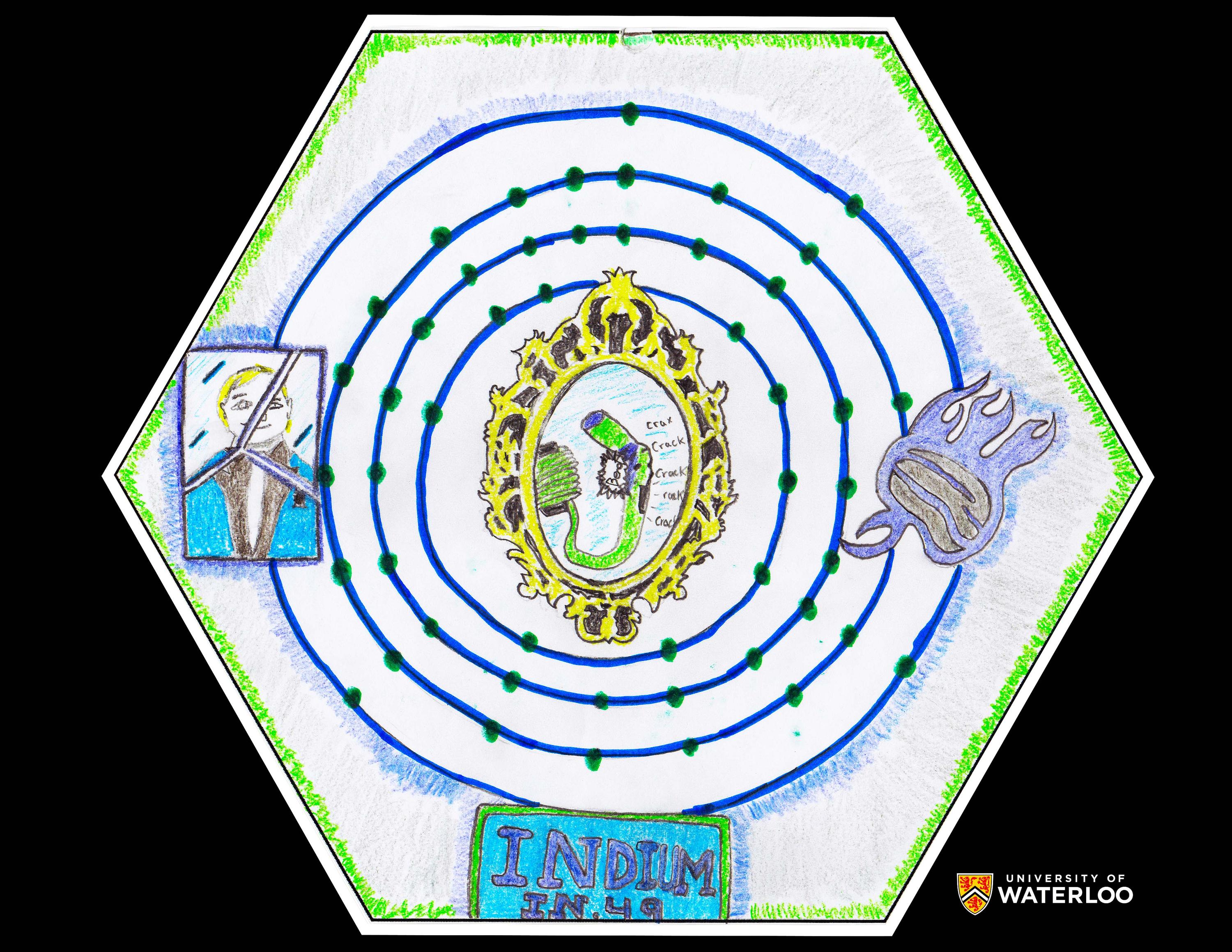
Regina, Saskatchewan, Canada
Teacher: Alex Hutchinson
Artist: ABE 10 Class
The large rings in the middle show the atomic structure of indium. On the right of the rings is an abstract drawing of blue flames. The blue represents indium's name origin after the colour indigo. In the middle of the rings is indium's metal snapping; a mirror is used as a border to make the picture appear more prepossessing and because indium can be used to make mirrors. On the left is a drawing of Ferdinand Reich, one of the discoverers of indium. Lastly, below the atomic structure is the name, symbol, and atomic number of indium.